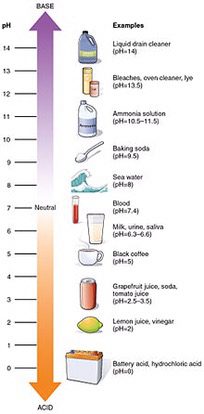
Why are acids dangerous? We use the pH scale to give one a relative scale of physical hazard to that of water, or H2O.
The pH scale has a range of 0 to 14, with water having a pH=7, or neutral. A pH of 1 indicates an acid (i.e. hydrochloric acid), whereas a pH of 14 (i.e. drain cleaner, lye) indicates an alkaline (base). Since it is a logarithmic scale measuring the level of Hydrogen ions, a change of one pH unit is a change in H- ions by a level of 10.
Pour water into water, and there is no reaction. However, pour one part acid (pH2) into one part of water (pH7) and you will see a reaction, resulting in ~pH3. A reaction can be as simple as slight fuming, or as violent as an explosion. The reaction occurs due to oxygen and hydrogen molecules rearranging themselves.
EPA defines a liquid being a corrosive acid as having a pH=2 or lower. EPA requires such waste to be accounted for on a hazardous waste manifest with an RCRA assigned characteristic code of “D002”. An acid with a pH=2 is quantum leaps more reactive than that of pH=5. Thus, it’s hazard level is much more severe.

For example, lemon contains citric acid. The acid is around pH=2.2, but it does not severely burn your tongue because the concentration of the acid is only around 6%. The same concept of reactivity occurs for alkaline liquids with pH=8 through 14. There are many chemicals that can hurt both your environment and health. Want to learn more about why are acids dangerous? Contact MLI Environmental for more info, and quotes for chemicals disposal.
